Introduction
Healthcare and technology IPOs were by far the most popular IPO sectors in the last 12 months, commanding 62% of the total offerings (healthcare 41%; technology 21%). These deals raised a total of $6.9 billion (Source: Renaissance Capital). Leading the way were biotech offerings, numbering 26 out of 34 healthcare deals.
As 2016 began, in Q1, the first (and only) deals to successfully price were biotechs: Corvus Pharmaceuticals (NASDAQ:CRVS), Syndax Pharmaceuticals (NASDAQ:SNDX), Proteostasis Therapeutics (NYSE:PTI), AveXis (NASDAQ:AVXS), Editas Medicine (NASDAQ:EDIT), and BeiGene, Ltd. (NASDAQ:BGNE). These were followed by more than 30 offerings after Labor Day across several sectors.
We have closely watched and continue to be particularly bullish on five biotech IPOs: AveXis, Clearside Biomedical (Pending:CLSD), Protagonist Therapeutics( Pending: PTGX), Reata Pharma (Pending:RETA), and bluebird bio (NASDAQ:BLUE). This group has outperformed in an extraordinary sector, and we plan to initiate or build on existing long positions in these companies heading into 2017.
AveXis
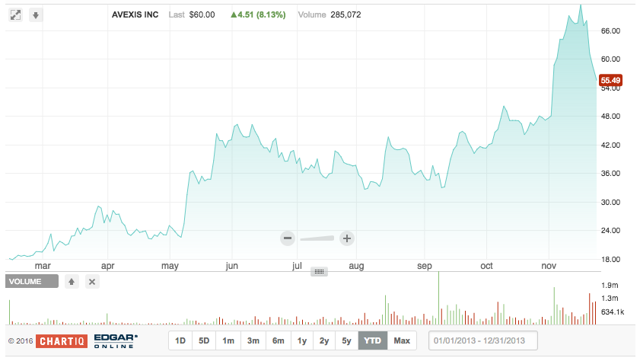
AveXis develops and sells novel treatments for patients suffering from rare, life-threatening neurological diseases. AVXS-101 is the company's initial product candidate, which is currently in Phase 1 clinical trials for treating spinal muscular atrophy (SMA) type 1. Q3 results highlighted progress within this trial, including AVXS-101's favorable safety profile. On November 1, 2016, AveXis announced a pivotal study of AVXS-101 in SMA Type 1, which will reflect a single-arm design and rely on the disease's natural history as a comparator.
On 2.10.2016, AVXS offered 4.8 million shares at $20/share, raising $96 million. Lead underwriters for the deal were Goldman Sachs and Jefferies LLC; underwriters were BMO Capital Markets and Chardan Capital Markets.
We initially liked this Goldman-led deal, following the success of gene-therapy company BGNE. Despite risks of some negative public perception to gene therapies, AveXis possessed novel technology. AVXS returned -9.8% on its first day and surged 207.4% in the aftermarket.
AveXis's IPO quiet period expired on 3.22.2016. It now reflects seven analyst ratings (six Buy; one Hold) with an average target price of $75. AveXis is currently priced at $59.44.
Clearside Biomedical
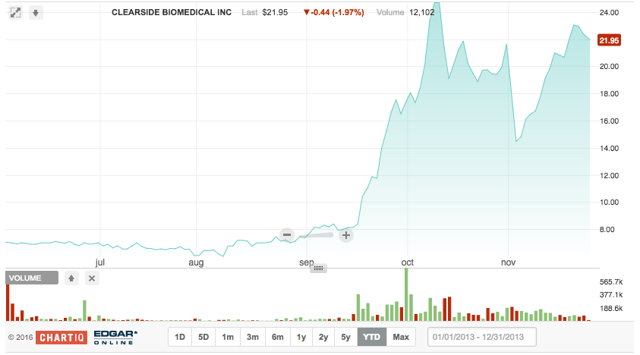
Clearside Biomedical develops a combination drug-device therapy for eye diseases. The company raised $50 million in its 6.1.2016 offering via 7.2M shares at $7 apiece. This was a slight change from the original size of 4M shares at a range of $14-16. Cowen & Company and Stifel were lead managers for the deal. The IPO did well, returning 3.6% the first day and 208.8% in the after-mkt.
On 4.26.2016, Clearside announced positive preliminary phase 2 results for treating macular edema associated with retinal vein occlusion (RVO). The company's lead product is in phase 3 trials.
The IPO quiet period for CLSD expired on 7.12.2016; since then, four analysts have initiated coverage - all with Buy ratings. The average target price of $25 is above its current price of $21.72.
CLSD is backed by three venture firms (Hatteras Partners, GRA Venture Fund, and RMI Investments), along with Santen Pharma (OTCPK:SNPHY).
Protagonist Therapeutics
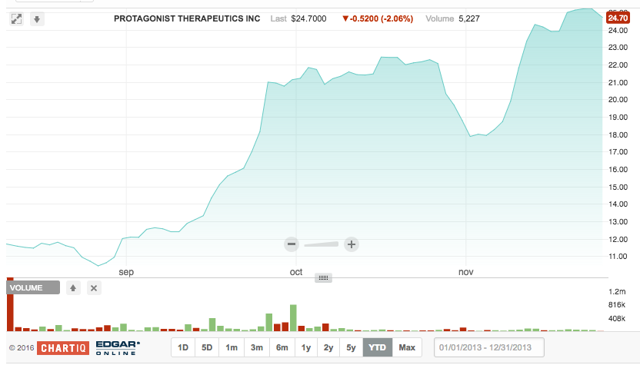
Protagonist Therapeutics is also in clinical stages, developing oral treatments for various gastrointestinal issues, including ulcerative colitis and inflammatory bowel disease. The company's oral peptide-based drugs aim to alleviate the need for people to have to undergo frequent injections.
Headquartered in Milpitas, California, Protagonist currently has three primary candidate drugs. The first, PTG-100, successfully completed a Phase 1 clinical trial in healthy volunteers in Australia. As noted in quarterly results, clinical data from this study was presented on October 17 in Vienna, Austria, at United European Gastroenterology Week. The company believes it is on track to initiate phase 2b study of PTG-100 by year-end 2016. This would be for moderate-to-severe ulcerative colitis patients.
On 8.10.2016, PTGX offered 7.5M shares at $12/share for a raise of $90,000,000. Underwriters for the IPO included Barclays, BMO Capital Markets and Leerink Partners. While we were initially cautious on Protagonist's IPO, given its early stages and stiff competition; we were proved wrong. Protagonist had a -2.5% first-day return and a whopping 115.6% return in the after-market.
Following the 9.20.2016 quiet period expiration, analysts began coverage. PTGX now has three ratings - all Buy. The average analyst price target is $27, above the current price of $25.12.
Reata Pharmaceuticals
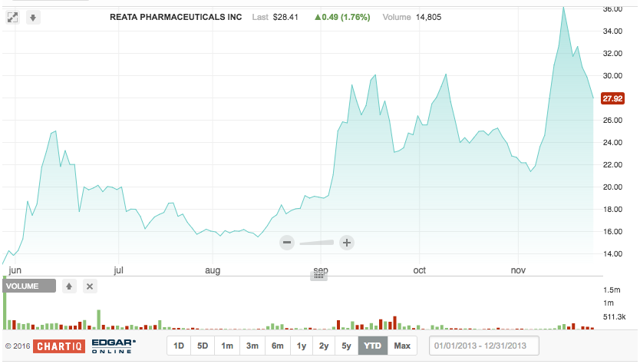
Reata Pharmaceuticals develops products that can modulate the activity of certain proteins involved in inflammation, oxidative stress and mitochondrial function. The purpose is to meet the medical needs of patients with various life-threatening diseases that currently have no treatment options. Reata is focused on product candidates that regulate certain proteins called transcription factors. Its leading product candidates include RTA-408 and bardoxolone methyl, a class of small molecules called antioxidant inflammation modulators.
On 5.25.2016, RETA offered 5.5M shares at a midpoint price of $11/share for a total raise of $60,500,000. Underwriters for the deal were Citigroup Global Markets, Cowen & Co., and Piper Jaffray & Co. As with PTGX, we were hesitant on the initial offering, yet were pleasantly surprised when the fledgling company took off. RETA returned 18.8% on its first day, followed by 113.6% in the after-market.
Since its IPO, Reata has moved forward with a new Phase 2/3 study for the treatment of chronic kidney disease ("CKD") caused by Alport syndrome. On October 6, 2016, a first patient was enrolled in Reata's international, randomized, double-blind, placebo-controlled Phase 3 trial for a lead candidate.
The quiet period on underwriter research for RETA expired 7.5.2016. Since then, three Wall Street analysts initiated coverage on the company - all with Buy ratings. Average target price is $40, well above the current $27.30.
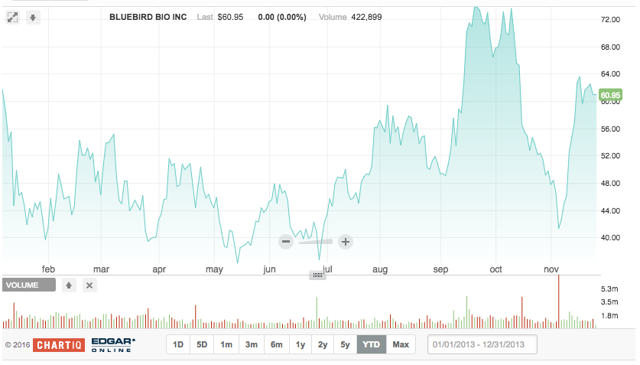
bluebird bio
Headquartered in Cambridge, Massachusetts, bluebird bio has operations throughout the U.S., as well as Europe. According to the company's website, bluebird bio is developing potentially transformative gene therapies for severe genetic diseases and T cell-based immunotherapies. The objective is to "provide patients hope for a better life in the face of limited or no long-term safe and effective treatment options". On 11.2.2016, bluebird released Q3 results, which highlighted the initiation of a HGB-207 Phase 3 study of LentiGlobin™ and the reception of the PRIME designation for LentiGlobin from the European Medicines Agency.
bluebird priced its NASDAQ IPO on 6.19.2013; the company offered 5.9M shares of common stock at $17.00 per share, before underwriting discounts, for a raise of $100.3 million. J.P. Morgan Securities LLC and BofA Merrill Lynch were joint book-running managers; Cowen and Company acted as lead manager, and Canaccord Genuity Inc. and Wedbush PacGrow Life Sciences were co-managers. The stock moved up >50% after the initial open.
We've watched bluebird for the past few years, writing about the lockup expiration and strong VC support. bluebird was backed by VC firm Third Rock Ventures and had previously raised $134 million in funding. Of 16 analyst ratings, 12 are Buy, three Hold, and one Sell heavily skewed in BLUE's favor. Avg. target price is $80.20, well above the current price of $61.30 (morning sessions 11.23.2016).
Conclusion
In 2015, we spoke publicly about our concern for the degrading quality of biotech offerings; however, our tune has changed with respect to the above companies. We are impressed with the favorable analyst reports and price targets, their IPOs and 2016 market performances, as well as impressive progress through clinical trials. Many of the group are supported by well-known and influential venture firms.
While the overall IPO market has been relatively weak in 2016, historically it has been a very strong year for biopharma deals. According to Forbes, in the past 20 years, excluding the present window, the market has only seen more than two dozen VC-backed IPOs in three vintage years.
Biopharma has outperformed even despite recent drug pricing volatility.
As noted above, we plan to initiate or build on existing long positions in these companies heading into 2017.