
Start Up Spotlight: OCON Medical Bets On A New 3D Copper Contraceptive Device
Print
19 January 2018
Catherine Longworth / MedTech Insight
Executive Summary
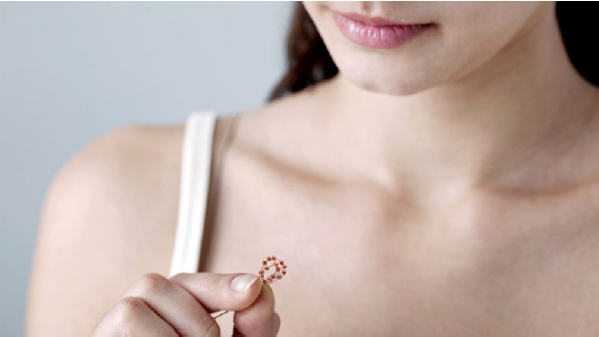 Israeli start up OCON Medical is blazing a trail in the field of women's health with a new hormone-free contraceptive device. The company's IUB – intrauterine ball consists of copper pearls that prevent pregnancy locally within the uterus for five years.
Israeli start up OCON Medical is blazing a trail in the field of women's health with a new hormone-free contraceptive device. The company's IUB – intrauterine ball consists of copper pearls that prevent pregnancy locally within the uterus for five years.
OCON Medical is on a mission to revolutionize a eld that is long overdue a shakeup with its IUB intrauterine ball, a copper contraceptive device that is designed to improve patient safety and quality of life compared to existing intrauterine devices.
OCON's technology originated in 2008 when company founder and senior gynecologist, Dr. Ilan Baram, attended the European Society of Contraception meeting in Spain. In one lecture a physician described his patients coming to him complaining of pain and bleeding after IUD insertion and showed videos of hysteroscopy procedures. Studies show that nearly 70% of women who have copper IUD's suffer from pain and bleeding after insertion but imaging was rarely being carried out.
 Baram began to image his patients and discovered that nearly all their IUDs were malpositioned or perforated to a certain extent. He could see the main reason for this was due to the T shape of the IUD not adapting to the shape of a woman's uterus.
Baram began to image his patients and discovered that nearly all their IUDs were malpositioned or perforated to a certain extent. He could see the main reason for this was due to the T shape of the IUD not adapting to the shape of a woman's uterus.
Baram then had a "eureka" moment, says Weinstein. "He thought to himself - why not make a ball shaped IUD to adapt to the uterus that would not malposition?" From that, the technology behind OCON Medical was born.
The company was officially founded in 2011 and within a span of half a year the team developed a final prototype device which is available on the market today.
The IUB works along the same principle as other intrauterine devices and prevents pregnancy locally - within the uterus and is effective for up to five years. The contraceptive effect comes mainly from the copper pearls which consist of a special shape memory alloy, which has been used in implants and stents for a long time. OCON launched the product in 2014, following the receipt of a CE mark and are now selling in over 23 countries across Europe and other parts of the world, with 50,000 women already using the product.
 Prior to joining OCON, Weinstein was CEO at device companies in cardiology and orthopedics and notes the differences he observed compared to women's health. "The attention span that these fields get at conferences and trade shows is overwhelmingly more significant than anything that women's health gets," he says. "But it's a hidden gem. Women's health will always be needed and there's always room for improvement and our company sees it as a wonderful opportunity to come in with a new technology in a field with no innovation."
Prior to joining OCON, Weinstein was CEO at device companies in cardiology and orthopedics and notes the differences he observed compared to women's health. "The attention span that these fields get at conferences and trade shows is overwhelmingly more significant than anything that women's health gets," he says. "But it's a hidden gem. Women's health will always be needed and there's always room for improvement and our company sees it as a wonderful opportunity to come in with a new technology in a field with no innovation."
Despite rapid innovation in many fields across medtech, women's health has seen little advancement. For many women across the world, contraceptive options are limited to the breakthrough products of yesteryear. "Women's health is considered a bit of an outcast field and rarely gets any attention," OCON Medical CEO Ariel Weinstein tells Medtech Insight. He said that the last major new IUD technology to be released to the public was Bayer'sMirena IUD, launched in 1991.
"We have a lot laying on our shoulders but we don't have a lot of competitors out there. Only recently do we see the field of women's health starting to grow but when we started it was barren land. We're the first in market so very interesting situation," he said. "All of these problems were underinvestigated for a long time because it was hard to ask women with an IUD to participate in a very unpleasant study involving placing a camera inside their uterus."
"This product is intuitive, simple and makes sense. It's tangible, it's very easy to understand," Weinstein says.
IUB reduces the risk of perforation, which affiicts about 50,000 IUD patients a year, as well as the risk of expulsion which happens up to 18.8% in young women. IUB "completely solved" the problem of malposition, he said.
 In 2011, after OCON completed development of the prototype the company realized the technology could be adapted for use as an intrauterine drug carrier platform. "What's nice about this product is its applied in a linear form inside a tube and then its deployed into the uterus and as it comes out from the deployment tube it turns into a sphere and coils into a bulb shape," he said. "So under this frame we can attach any substance we wish which is our proprietary technology. We can attach all types of substances to this frame and all types of adaptations can be made for applications."
In 2011, after OCON completed development of the prototype the company realized the technology could be adapted for use as an intrauterine drug carrier platform. "What's nice about this product is its applied in a linear form inside a tube and then its deployed into the uterus and as it comes out from the deployment tube it turns into a sphere and coils into a bulb shape," he said. "So under this frame we can attach any substance we wish which is our proprietary technology. We can attach all types of substances to this frame and all types of adaptations can be made for applications."
An IUD device like this has not been developed before "probably because only 50% of the population can benefit from this and also because there's never been an appropriate drug carrier technology," Weinstein said.
The company has also started developing two additional pipeline products, Sphera a hormone eluting spherical IUD based on the IUB platform and a second one which is intended to treat abnormal uterine bleeding. First-in-human studies for this technology will begin in the next couple of months, Weinstein says.
"We're a small company and we see this as a nice breakthrough – there has never been an attempt to use the uterus to deliver drugs," Weinstein saidOCON has also begun work on its next product which will be for a range of indications including IVF, hormone therapy, cancer treatment. A US study of the IVF product will start in 2018 and the rst-in-human study of a hormonal IUD will begin by early 2019.
All Portfolio
MEDIA CENTER
-
The RMI group has completed sertain projects
The RMI Group has exited from the capital of portfolio companies:
Marinus Pharmaceuticals, Inc.,
Syndax Pharmaceuticals, Inc.,
Atea Pharmaceuticals, Inc.

