Antisense drugs interfere with the communication process that tells a cell to produce an unwanted protein. Vaccines, particularly, therapeutic cancer vaccines, are another example of how the immune system is being harnessed to fight disease.
• A monoclonal antibody for the treatment of asthma.
The Human Genome
Inside every human body are about 25,000 genes—each responsible for a specific protein. A person’s genes tell their body to produce all the enzymes, hormones, antibodies and
other proteins needed to make the body function. If one of the genes is missing or defective, the body will not have the proteins it needs to function properly and may have proteins that actually cause disease. The modern tools of biotechnology—coupled with computer technology’s ability to analyze massive amounts of data quickly—help biopharmaceutical scientists determine which genes or proteins are defective and are being used to develop new treatments across a range of therapeutic areas.
Manufacturing Complexities Most biologics are very complex molecules and cannot be fully characterized by existing science. For this reason, they often are characterized by their manufacturing processes. Given the complexity of many biologics, the manufacturing processes are likewise complex and very sensitive. Slight changes in temperature or other factors can impact the final product and affect how it works in patients. Changes in the manufacturing process or facility may require clinical studies to demonstrate safety, purity and potency.
Monoclonal Antibodies: Targeted Cancer Therapy
An approved monoclonal antibody (mAb) for the treatment of cancer targets the epidermal growth factor (EGFR) that is linked to the growth and development of many types of cancer. Originally the mAb was approved for the treatment of EGFRexpressing metastatic colorectal cancer. Additional studies have found that the presence or absence of a certain gene mutation could predict the patients’ response to treatment. Patients without the gene mutation—about 65% of patients—are most likely to benefit from treatment.
Over the past decade, biologics have accounted for one-third of new medicine approvals.
Advancing Biomedical Science
Over the past decade, a wave of scientific advances and new technologies have dramatically changed how medicines are discovered. Greater knowledge of how diseases work at the genetic and molecular level has allowed researchers to pursue new targets for therapy and better predict how certain biopharmaceuticals will affect specific subpopulations of patients.
• Bioinformatics—Bioinformatics use systems and mathematical models to advance the scientific understanding of living systems. At its simplest level, bioinformatics involves the creation and maintenance of biological databases, including DNA sequences. Bioinformatics also includes calculation tools. These tools can decipher the molecular pathways of disease, find patterns in the way genes respond to drugs, interpret the three-dimensional structure of important proteins, and enable the computer-aided design of new drugs.
• Biomarkers—Every disease leaves a signature of molecular “biomarkers” in our body—genes that turn on and off or proteins released into the bloodstream. Biomarkers measured in blood and other samples can tell us the state of our health and how we might respond to treatment. They
are powerful tools that can detect certain diseases at their earliest stages before symptoms appear, when they are most treatable. The identification of biomarkers is the first step in developing a personalized medicine.
• Molecular Targeting—The idea behind molecular targeting is to design drugs that specifically attack the molecular pathways that cause disease, without disrupting the normal functions in our cells and tissues.
• Nanotechnology—You can’t see it, but soon it will be everywhere. Nanotechnology is the science of building microscopic devices at the molecular and atomic levels. In medicine, nanotechnology may also be used to help diagnose and treat diseases. For example, tiny gold-coated “nanoshells” could act like smart bombs, zeroing in on a tumor, entering cancer cells, and lying in wait until an infrared beam or radio wave signals the particles to release an intense, deadly dose of heat energy that destroys the cancer cells.
• Personalized Medicine—The sequencing of the human genome produced a “map” of the human genes in DNA. This new genetic knowledge opens up the possibility of developing “targeted” therapies for people with specific gene sequences, and it can help physicians choose the best treatments based on individual genetic, lifestyle and environmental factors. Additionally, researchers are developing genetic tests that can tell if we are susceptible to certain diseases.
Key Biologic Medicines Approved in the Last Decade
• The first genetically engineered antibody approved to prevent the formation of new blood vessels that provide tumors with oxygen and nutrients—a process called angiogenesis. The medicine was approved for the treatment of metastatic colorectal cancer. Using angiogenesis as an approach to fight cancer was first discussed more than 30 years ago. In 1989, biopharmaceutical company scientists discovered a key growth factor influencing the process that led to the discovery and development of the medicine.
• A first-in-class human monoclonal antibody that targets the cytokines interleukin-12 (IL-12) and interleukin-23 (IL-23). IL-12 and IL-23 are naturally occurring proteins that are believed to play a role in the development of psoriasis. The medicine is delivered by injection four times a year after two initial doses within four weeks.
• A recombinant vaccine for the prevention of human papillomavirus (HPV) which can lead to cervical and other cancers. The vaccine was approved for the prevention of cervical diseases caused by human papillomavirus (HPV) types 16 and 18 for use in females ages 10 to 25. In clinical trials, the vaccine was shown to be 93 percent effective in preventing cervical pre-cancers associated with HPV 16 and 18 in women with no prior exposure to HPV of the same types.
• The first in a new therapeutic class called autologous cellular immunotherapy. Approved for the treatment of metastatic, castrate-resistant (hormone-refractory) prostate cancer, the medicine was designed to induce an immune response against prostatic acid phosphatase (PAP), an antigen expressed in most prostate cancers. Each dose is manufactured using the patient’s own immune cells from the blood. To enhance their therapeutic response against the cancer, the immune cells are then exposed to the PAP antigen and linked to an immune stimulating substance. when this process is complete, the patient’s cells are returned intravenously to the patient to treat the cancer.
•
The first new medicine approved to treat adults with active lupus in over 50 years and the first in a new class of biologic therapies called BLyS-specific inhibitors. Researchers identified a naturally occurring protein in the human body called B-lymphocyte stimulator (BLyS). Clinical studies have shown that there is a connection between higher levels of BLyS and lupus disease activity in some people. This new monoclonal antibody delivered through an intravenous (IV) infusion, binds to BLyS and prevents it from stimulating B cells. Adding this therapy to other lupus treatments may help reduce the abnormal immune system activity that contributes to disease activity in lupus.
• The first in a new class of antibody-drug conjugates (ADC) which utilizes a monoclonal antibody to direct a therapeutic drug to target the cancer cells. It was approved to treat Hodgkin lymphoma and systemic anaplastic large cell lymphoma (ALCL), a rare type of lymphoma that represents only 3 percent of all non-Hodgkin lymphomas. ADCs combine a monoclonal antibody and a therapeutic drug, where the antibody directs the therapeutic to target the cancerous cells. It is also the first FDA-approved drug for Hodgkin lymphoma in more than 30 years and the first to specifically treat ALCL. It is composed of an anti-CD30 monoclonal antibody and a microtubule disrupting agent and releases its therapeutic drug once inside the CD30-expressing tumor cells.
RNA Interference (RNAi)
RNA interference therapeutics are an exciting new frontier for the development of novel therapies for patients, especially patients with genetic disorders. There are several RNAi therapies in clinical trials which have demonstrated potential in treating certain neuromuscular disorders, such as Duchenne Muscular Dystrophy (DMD). DMD is a genetic disorder impacting 1 in 3,500 newborn boys and is the most severe form of muscular dystrophy in childhood. One RNAi targeted therapeutic in development seeks to restore the function of dystrophin. Early clinical trials of the drug have demonstrated significantly improved dystrophin expression as well as improvement in DMD patients’ ability to walk.
Medicines in the Future
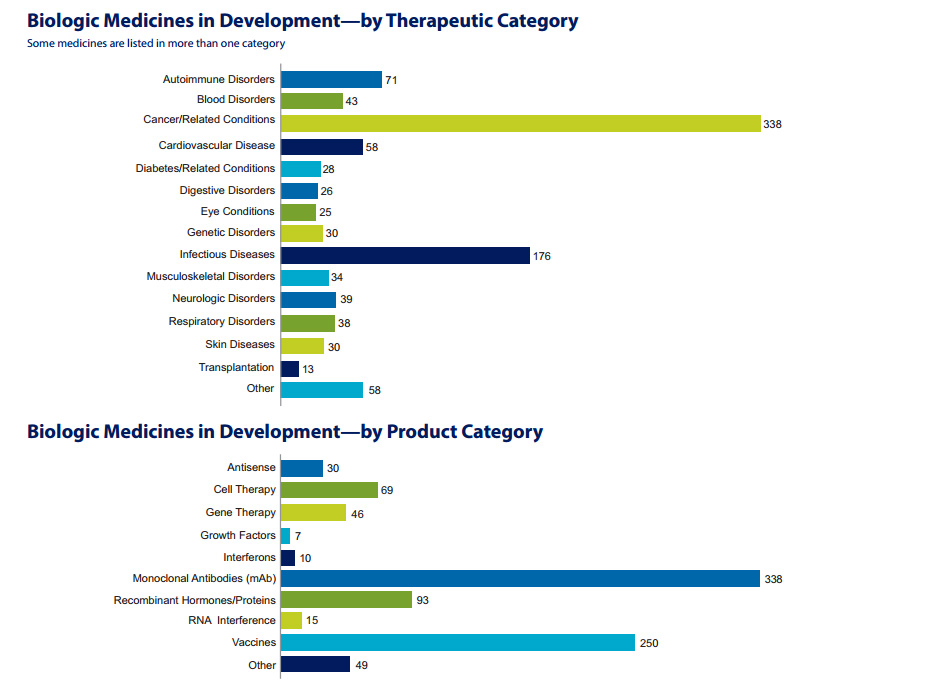
Building on the impressive progress to date, the 907 medicines and vaccines listed in the PhrMA report, Biologic Medicines in Development, represent the next exciting frontier of biopharmaceutical research.
The report finds that the greatest amount of research is in monoclonal antibodies (mAbs), with 338 separate mAbs in development, and vaccines, with 250 vaccines in clinical trials or under review at FDA.
Analysis: Cancer and Infectious Diseases and Technologies
The report on biologics finds that a great deal of research is focused in two diseases areas—cancer and infectious diseases. Within these categories, biopharmaceutical researchers are investigating several different techniques and technologies to treat and prevent disease.
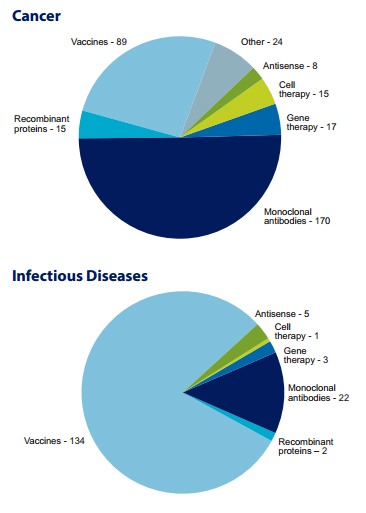
MAbs were first explored as a therapeutic option as a result of scientific breakthroughs that occurred in the mid-1970s and early-1980s. By 2013, a total of 33 mAbs were approved in the United States. Because mAbs allow targeting of unhealthy cells without harm to healthy cells, they have been particularly important in fighting cancer, and more recently, show great promise for autoimmune diseases, such as rheumatoid arthritis. Vaccines have historically been used as a preventative tool in infectious diseases, such as pneumonia, HIV and smallpox. But today, vaccines are also being used as therapies for cancer and other diseases.
Below are examples of some of the exciting new biologic medicines in the pipeline:
Monoclonal Antibodies (mAb)
• A mAb designed to block the IL-13 cytokine, a protein messenger between cells that triggers inflammation. Blockage of IL-13 may reduce the risk of asthma and other respiratory diseases.
• A mAb that targets B-cells that cause the immune system to turn against itself and produce antibodies against the body’s own cells and tissue.
• A mAb for the treatment of psoriasis is an engineered human antibody to interleukin-17 (IL-17), a key cytokine involved in inducing and mediating inflammation associated with psoriasis.
• A mAb directed against interleukin-6 (IL-6) alpha, a signaling protein involved in the regulation of immune and inflammatory responses associated with rheumatoid arthritis. The mAb interrupts the inflammatory signaling cascade of IL-6 by blocking its binding to a certain receptor necessary for inflammatory cascade.
• A mAb for potential use in the regeneration of corticospinal tract fibers resulting from an acute spinal injury.
The antibody neutralizes a protein that inhibits growth of spinal fibers.
Antisense
• A third-generation antisense medicine in development for the treatment of lymphoma inhibits production of a specific protein which regulates many key genes important in cancer growth—angiogenesis, cell metabolism, cell proliferation, cell death and cell invasion. An overexpression of the protein in tumors results in resistance to treatment. By reducing the amount of the protein in cancer cells, the antisense medicine may be able to enhance the effectiveness of current anticancer treatment.
Therapeutic Vaccines
• A virus-based therapeutic vaccine in development for the treatment of melanoma is genetically-modified to replicate selectively in tumor cells and express a gene for an immune-stimulating protein. It is injected directly into the tumor where it replicates and spreads within the tumor, causing the death of cancer cells and stimulating the immune system to destroy cancer cells.
• An immunotherapeutic designed to train the immune system to recognize and eliminate cancer cells in a highly specific way. The medicine is a combination of tumor antigens, delivered as recombinant proteins, and a proprietary adjuvant (an agent that modifies another substance in its action) to stimulate the immune response to cancer cells. It is intended to only affect cancer tissue and not harm normal tissue.
RNAi—RNA Interference
• An rNAi-targeted therapeutic in development seeks to restore the function of dystrophin. Early clinical trials of the medicine have demonstrated significantly improved dystrophin expression as well as improvement in DMD patients’ ability to walk.
Stem Cell Therapy
• Researchers are exploring transplantation of a patient’s own bone marrow cells into damaged heart tissue to regenerate heart tissue. It is believed that a patient’s immune system will not attack the newly transplanted cells because they are native to the patient.
Gene Therapy
• A gene therapy employs an adeno-associated virus (AAV) as a vector to deliver to the gene neurturin, which has been found to restore cells damaged in Parkinson’s patients and protect them from further degeneration.
Encouraging Continued. Biopharmaceutical Innovation
The development of new biologics is a long, complex and costly endeavor. It takes about 10–15 years, on average, to bring a medicine through the discovery and clinical trial phases to patients, and the average R&D investment for each new medicine is $1.2 billion, including the cost of failures.
Another report on medicines in the pipeline found that more than 5,000 potential new medicines—which may become available to U.S. patients—are in the pipeline globally—in large part funded by the more than $500 billion invested in research and development (r&D) since 2000 by PhrMA member companies. These promising candidates build on the more than 300 medicines that have been approved by the FDA in the last decade.
In order to realize the full potential of biologics, it is essential that the United States maintain strong policy and regulatory environments that help foster the discovery and development processes.
Human Clinical Trials
Phase I—Researchers test the biologic/drug in a small group of people, usually between 20 and 80 healthy adult volunteers, to evaluate its initial safety and tolerability profile, determine a safe dosage range, and identify potential side effects.
Phase II—The biologic/drug is given to volunteer patients, usually between 100 and 300, to see if it is effective, identify an optimal dose, and to further evaluate its short-term safety.
Phase III—The biologic/drug is given to a larger, more diverse patient population, often involving between 1,000 and 3,000 patients (but sometime many more thousands), to generate statistically significant evidence to confirm its safety and effectiveness. They are the longest studies, and usually take place in multiple sites around the world.
The Promise of PDUFA V
An efficient, consistent and predictable science-based regulatory system is critical to providing innovative new medicines to patients. The recently reauthorized Prescription Drug User Fee Act (PDUFA V) will help foster timely patient access to new medicines, enhance the FDA’s regulatory science capacity and encourage future innovation while strengthening the Agency’s high safety standards. It provides for increased scientific communication between FDA and drug sponsors during the regulatory review of new medicines which has the potential to increase first-cycle approval rates and bring new medicines to patients faster. PDUFA V will also advance regulatory science through resources to validate the use of new scientific tools (such as pharmacogenomics and biomarkers) and meta-analyses that will help decrease drug development time.
The Future of Research Should Be Protected by Adequate Incentives for Innovation; 12-Years of Data Protection is Critical for Patients
A biosimilar is similar to—but not the same as—an innovator biologic. A pathway for the approval of biosimilars in the United States was included in the Patient Protection and Affordable Care Act of 2010. The pathway, which received broad bipartisan support in both chambers of Congress, struck an appropriate balance between promoting increased competition and providing adequate incentives to support continued innovation of new treatments and cures through a 12-year period of data protection. This is critical to spurring the investment in research and development needed to seize the extraordinary opportunities for medical advances against our most costly and challenging diseases and the resulting jobs supported across the U.S. economy.
U.S. pharmaceutical companies account for 80% of the world’s R&D in health care biotechnology.
The Biomedical Research Ecosystem
The collaborative ecosystem that exists in the United States between the government, academia, and biopharmaceutical companies is among our greatest strengths in moving medical advances forward and has helped position the United States as the worldwide leader in biopharmaceutical innovation.
Biopharmaceutical companies today are engaging in a wide range of partnerships in new and promising ways. Such partnerships include unrestricted research support, academic drug discovery centers and pre-competitive research centers.
As scientists have probed deeper into the causes and signs of disease the amount of information that is available has exploded, but making sense of all the data is a colossal undertaking that no single individual or institution can handle alone.
As a result, biopharmaceutical research companies are working with other companies, academic medical centers, the government, and in some cases non-profit organizations to share, organize and make sense of huge volumes of information. In some instances, they are working together in innovative ways to share information to advance progress against disease.
An example of this kind of collaboration is the Alzheimer’s Disease Neuroimaging Initiative, which includes federal agencies, non-profit organizations, and biopharmaceutical industry members. The goal of the Initiative is to track Alzheimer’s disease progression, establish quality standards and validate biomarkers to be used in clinical trials. Data collected are made available at no cost to researchers when designing clinical trials and research projects.
Corporate Venture Capital Investments are Helping Advance Biotechnology R&D
Another evolving aspect of the biomedical research ecosystem relates to the investment necessary to support emerging biopharmaceutical companies. Historically, venture capital and other forms of private capital have been key sources of financing for start-up and emerging biopharmaceutical
companies.
But, as traditional venture capital has recently declined due to several factors including regulatory challenges and concerns about coverage and payment of new medical innovations, the corporate venture arms of established biopharmaceutical companies have become an increasingly important source of capital to help fill this gap. Between 2010 and 2011, biopharmaceutical companies invested nearly $700 million in venture capital in biotechnology start-ups, according to a 2011 MoneyTree report. And corporate venture activity is on the rise.
According to a recent report by the Boston Consulting Group, 63% of the 30 largest biopharmaceutical companies currently participate in corporate venture capital investments—up from 50% in 2007.
Summary
The 907 biologic medicines and vaccines currently in development offer great hope for patients, building upon the significant progress in the last few decades across a wide array of diseases. America’s biopharmaceutical research ecosystem is uniquely positioned to deliver on the great
promise of science if supported by policy and regulatory environments that encourage continued innovation.



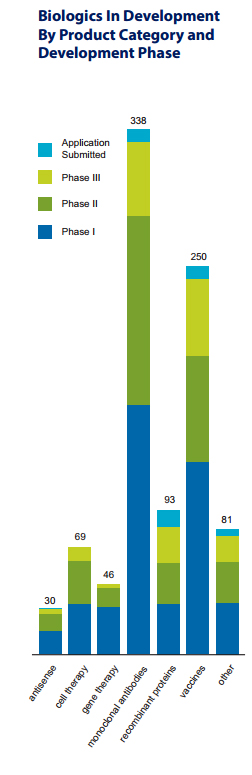 microorganisms. Like small-molecule drugs, some biologics are intended to treat diseases and medical conditions. Other biologics are used to prevent or diagnose disease. Examples of biological products include but are not limited to:
microorganisms. Like small-molecule drugs, some biologics are intended to treat diseases and medical conditions. Other biologics are used to prevent or diagnose disease. Examples of biological products include but are not limited to: 




