
New Report Highlights a Decade of Innovation in Rare Diseases
Print
11 March 2015
Stephanie Fischer / PhRMA
Despite Scientific Challenges, Biopharmaceutical Researchers Making Progress against Rare Disease
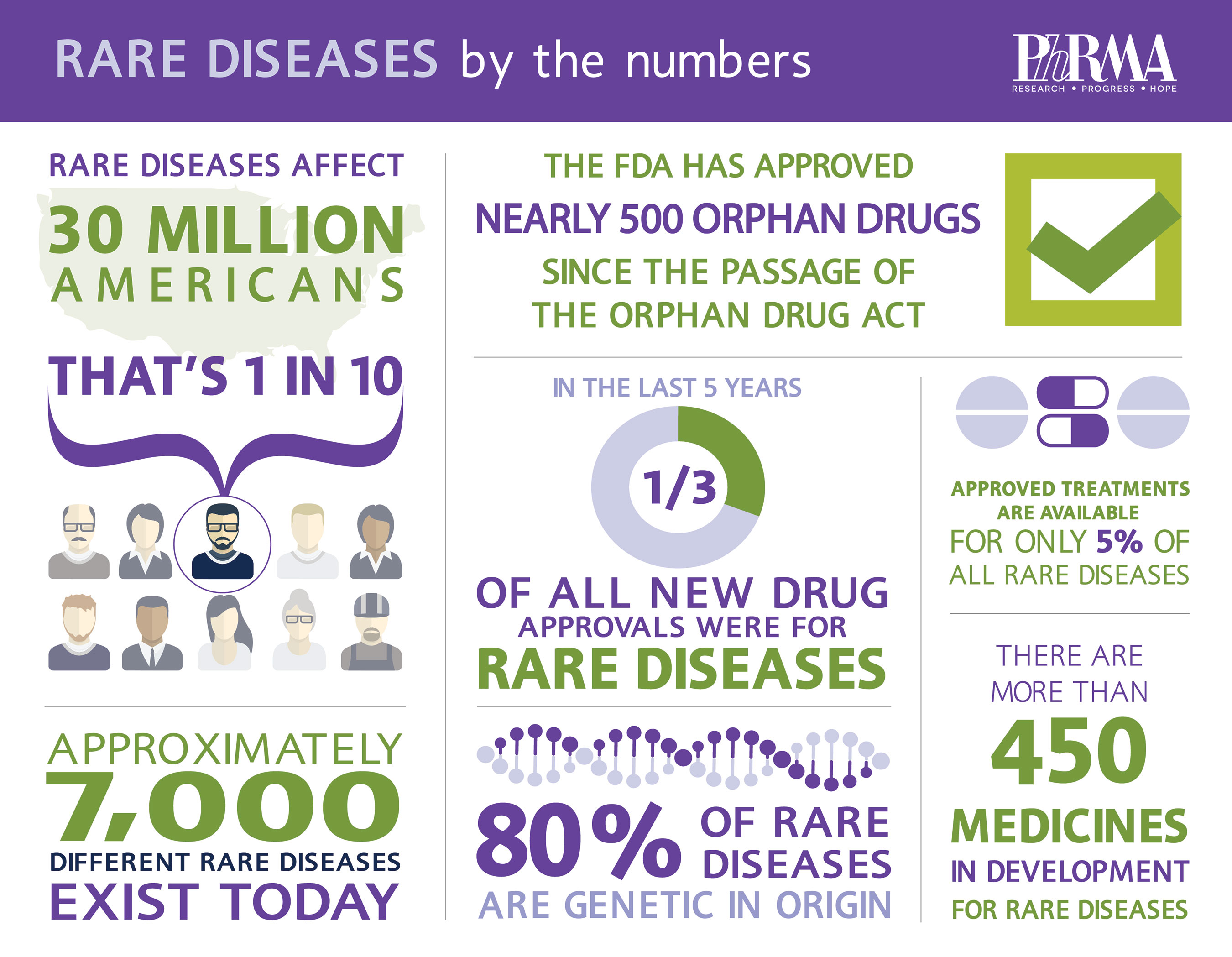
While any given rare disease affects fewer than 200,000 people in the U.S., rare diseases cumulatively affect 1 in 10 Americans and an estimated 350 million people worldwide.
On Saturday, there were efforts around the world to mark Rare Disease Day. This year’s theme, Living with a Rare Disease, put the focus on the daily impact on patients, families and caregivers:
“Typically chronic and debilitating, rare diseases have enormous repercussions for the whole family. Living with a rare disease becomes a daily learning experience for patients and families. Though they have different names and different symptoms, rare diseases impact the daily lives of patients and families in similar ways.”
One of the statistics often cited in the rare disease community is that 95% of rare diseases do not yet have a treatment approved by the Food and Drug Administration (FDA). The development of new therapies for rare diseases is challenging due in part to a lack of understanding about underlying biological mechanisms and clinical progression for many of them. Designing clinical trials, recruiting patients, and gaining statistically-significant conclusions can also be difficult, with small patient populations scattered around the world.
Progress against Rare Diseases
Despite these challenges, we are making progress against rare disease. There have been nearly 500 orphan approvals by FDA since the passage of the Orphan Drug Act of 1983, including both new drugs and new indications for orphan diseases. 233 of these approvals were in the last decade, including 46 in 2014 alone. Of the 41 new drugs approved by FDA’s Center for Drug Evaluation and Research (CDER) last year, 17 treat rare disease. A robust pipeline of more than 450 medicines in development for rare disease offers hope that this trend will continue.
A new report, A Decade of Innovation in Rare Diseases, highlights advances made in the last ten years against a variety of rare diseases, providing new treatments for patients who need them. For example, new treatment options for pulmonary arterial hypertension allow patients to maintain an active lifestyle with reduced risk of serious heart events, and breakthroughs in the underlying cause of hereditary angioedema have led to preventative and acute treatment options for patients who previously had no medications approved specifically for their disease. In addition, the next generation of targeted therapies can improve the quality of life and extend the lives of patients with chronic myelogenous leukemia and chronic lymphocytic leukemia.
Another example is the dramatic progress in the treatment of cystic fibrosis. Advances in addressing both the symptoms and he underlying causes of the disease have resulted in therapies to improve and extend the life of cystic fibrosis patients. A therapy approved in 2012 targets particular genetic mutations that cause cystic fibrosis, and has been used as an example of the very real promise of personalized medicine.
Work Remains to Address Unmet Medical Need of Rare Disease Patients
While we celebrate the positive impact of these scientific advances for patients, there is much work to do for the millions of patients with rare diseases that do not yet have FDA-approved treatments. Researchers in biopharmaceutical companies, government, and academia are building on recent breakthroughs and exploring new targeted mechanisms for treatment, often in collaboration with patient advocacy organizations. It is critical that public policy and regulation foster the research and development of new treatment options for rare disease patients.
All Portfolio
MEDIA CENTER
-
The RMI group has completed sertain projects
The RMI Group has exited from the capital of portfolio companies:
Marinus Pharmaceuticals, Inc.,
Syndax Pharmaceuticals, Inc.,
Atea Pharmaceuticals, Inc.

