
Tufts: The vaccine pipeline is soaring and global sales could hit $40B by 2020
Print
20 July 2015
Marie Powers / BioWorld
The vaccine product pipeline has never been fuller, with more than triple the number of candidates in development today than in 2005 and global sales on track to reach $40 billion in five years, according to the July/August Impact Report from the Tufts Center for the Study of Drug Development (CSDD).
The number of vaccine candidates in clinical trials worldwide more than doubled between 2002 and 2013, from 102 to 250, and grew from just 77 that were in human studies in 1998, according to the study. The pipeline is seeing a payoff, as well: Global vaccine sales rose at an average annual rate of 11.5 percent between 2005 and 2014 – nearly double the 6 percent average annual growth rate for total pharmaceutical sales. Global vaccine sales were estimated to be $29.6 billion in 2014, according to the report.
The analysis was conducted by Ronald Evens, adjunct research professor at Tufts CSDD and Tufts University School of Medicine and adjunct professor in the Thomas J. Long School of Pharmacy and Health Sciences at University of the Pacific, using data from company reports, periodic biotechnology reports from the Pharmaceutical Research and Manufacturers of America, IMS sales data, and FDA and Tufts CSDD databases.
Evens cited a variety of factors driving vaccine sales growth, including disease characteristics such as prevalence, annual recurrence, resistant bacterial strains and epidemic and pandemic potential. Factors propelling vaccine R&D include bioterror countermeasures, increased health care spending in the developing world, vaccines targeting unmet medical needs and new technologies. The emergence of vaccine candidates for cancer, included in the analysis, also swelled the R&D numbers.
Five vaccine products reached blockbuster status in 2014, as indicated in graph, "Vaccine sales worldwide by company, 2014," below. Overall, 122 drug and biotech products had sales exceeding $1 billion in 2014, according to Evens.
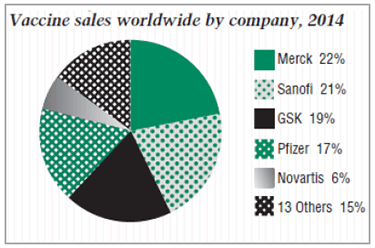
Combined sales of Pfizer Inc.'s Prevnar 7 (pneumococcal 7-valent conjugate) and more commonly used Prevnar 13 (pneumococcal polysaccharide conjugate vaccine [13-valent, adsorbed]), took the top spot, with $4.64 billion in sales – nearly $4.5 billion of those for Prevnar 13, according to Cortellis Competitive Intelligence. New York-based Pfizer gained Prevnar in its 2009 takeover of Wyeth, of Madison, N.J., for $68 billion. Prevnar 13 was approved by the FDA a year later and extended to adults last year. (See BioWorld Today, Jan. 27, 2009, and Feb. 25, 2014.)
Although 18 companies market vaccines, five – Merck and Co. Inc. of Kenilworth, N.J., Paris-based Sanofi SA, Glaxosmithkline plc (GSK), of London, Novartis AG, of Basel, Switzerland, and Pfizer – dominated the category during the last decade, accounting for more than 80 percent of global sales.
But the market remains robust for new entrants. More than 150 companies conducted vaccine trials in 2013 – constituting 28 percent of biotechnology products in development that year, according to the report – and vaccines presently in development aim to treat and prevent more than 40 infectious diseases. They include illnesses such as dengue fever, malaria, HIV and Ebola virus that have ravaged large geographical areas in emerging territories.
"The size of the commitment in research was surprising, as was the extremely large number of biotech companies engaged in this area of research," Evens told BioWorld Today.
'PHARMA COMPANIES SWOOP IN, ACQUIRE' LATE-STAGE ASSETS
Continued interest and funding of vaccine development was particularly significant in light of the challenges, and some early failures, in cancer vaccine development and the potential to suck resources from the space.
"I was surprised that there's been no downturn in the cancer vaccine research area," Evens admitted. In fact, the vaccine market has begun to settle into three "buckets" of companies: those developing conventional vaccines for new indications, such as Ebola virus; those focused on immuno-oncology; and those working in both areas, "although the latter is the smallest areas, by far," he added.
And M&A is just as active in the vaccine sector as in other areas of drug development, Evens said. Six companies – all pharmas – completed 16 acquisitions over the past 10 years for a total acquisition cost of more than $104 billion, as indicated in chart, "Vaccine company acquisitions, 2005 – 2015," below. In addition to Pfizer's take-out of Wyeth, Astrazeneca’s $15.6 billion pick-up of Medimmune Inc. also was owed in large part to the Gaithersburg, Md.-based company's vaccine products and pipeline. (See BioWorld Today, April 24, 2007.)
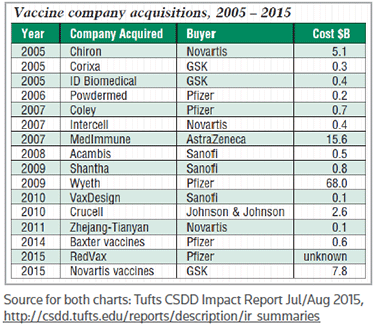
Data compiled by Thomson Reuters Recap confirmed the trend cited in the Tufts CSDD report, showing an uptick in 2013 M&A deals for therapeutics targeting infectious disease, including vaccines. Among the largest 2013 deals in the space were the $250 million acquisition of Inviragen Inc., of Fort Collins, Colo., by Osaka, Japan-based Takeda Pharmaceutical Co. Ltd., GSK's buyout of Swiss firm Okairos AG for €250 million (US$323.8 million), and Osaka, Japan-based Mitsubishi Tanabe Pharma Corp.'s grab of Canadian vaccine developer Medicago Inc. for $357 million. (See BioWorld Today, May 9, 2013, May 30, 2013, and July 15, 2013.)
That momentum carried over into 2014 and into the first quarter of this year, which saw GSK pay $190 million to acquire Swiss vaccine developer Glycovaxyn AG. (See BioWorld Today, Feb. 12, 2015.)
Evens expects to see more. Until recently, the need to stem infectious diseases in the developing world was underappreciated, he said, but that's changing as governments and major foundations, such as the Bill and Melinda Gates Foundation, plow money into these areas and companies make major commitments to the space.
"A lot of these companies are very independent, but as they move products into phase II and phase III we see the pharma companies swoop in to acquire them," he said. "And that's typical of biotech. They're on their own during the early stages, and then there is the big decision of what to do once the research gets downstream. Many want to be acquired."
Nevertheless, some biotechs have taken their vaccines all the way to market, Evens pointed out, citing Protein Sciences Corp. and its Flublok product. The Meriden, Conn.-based company recently disclosed top-line data showing its Flublok Quadrivalent – the quadrivalent version of FDA-approved trivalent Flublok shot – outperformed a traditional flu vaccine last season. (See BioWorld Today, July 1, 2015.)
"Bottom line, there's been an excellent commitment by the biopharma industry to address a broad scope of unmet medical needs related to vaccines for infectious disease and cancer," Evens said. "The future looks exciting."
Editor's note: For more information about the report, go to: http://csdd.tufts.edu/reports/description/ir_summaries
All Portfolio
MEDIA CENTER
-
The RMI group has completed sertain projects
The RMI Group has exited from the capital of portfolio companies:
Marinus Pharmaceuticals, Inc.,
Syndax Pharmaceuticals, Inc.,
Atea Pharmaceuticals, Inc.

