
Progress reviewed for new treatments that target osteoporosis
Print
18 April 2016
Peter Winter / BioWorld
The world's population is aging and that statistic means the incidence of chronic and serious life-threatening diseases is also on the rise. One of those conditions that challenge health care systems around the world is osteoporosis, a skeletal disease, which brings with it significant economic and health burdens.
The disease causes almost 9 million fractures annually – and worldwide, one in three women over the age of 50 will experience osteoporotic fractures, as will one in five men over 50, according to the International Osteoporosis Foundation. There is an ongoing need for effective treatments in addition to the current bisphosphonate therapies such as Fosamax (alendronate sodium, Merck & Co. Inc.), Zometa (zoledronic acid, Novartis AG) and Boniva (ibandronate sodium, Genentech Inc. and Glaxosmithkline plc).
Among the presentations on hormone-related conditions at the Endocrinology Society annual meeting (ENDO), the progress of promising new medicines targeting osteoporosis were discussed.
NEW TARGETS
Research advances in the understanding of the molecular regulators of bone remodeling have led to the identification of new targets for therapeutics such as monoclonal antibodies (MAbs).
At ENDO, Amgen Inc., of Thousand Oaks, Calif., and UCB SA, of Brussels, reported detailed phase III results showing that romosozumab, an inhibitor of protein sclerostin, demonstrated a statistically significant increase in hip bone mineral density (BMD) and strength compared with teriparatide in postmenopausal women with osteoporosis transitioning from bisphosphonate treatment. The data, from the STRUCTURE (STudy evaluating effect of RomosozUmab Compared with Teriparatide in postmenopaUsal women with osteoporosis at high risk for fracture pReviously treated with bisphosphonatE therapy) study, showed the percent change from baselines in BMD at the total hip through month 12 was significantly greater with romosozumab (2.6 percent) vs. teriparatide (-0.6 percent), for a mean difference of 3.2 percent (p <0.0001). The trial enrolled 436 women, average 72 years of age, with postmenopausal osteoporosis, a history of nonvertebral fracture after the age of 50, or vertebral fracture and treatment with bisphosphonate therapy for a minimum of three years prior to transitioning to romosozumab or teriparatide therapy. (See BioWorld Today, April 5, 2016.)
"These findings are especially important because they show romosozumab provided significant improvements in hip bone strength in a population that remained at high risk of fracture despite bisphosphonate therapy," noted Bente Langdahl, professor at the department of endocrinology and internal medicine at the Aarhus University Hospital in Denmark and STRUCTURE investigator.
Amgen is no stranger to MAbs and their use in osteoporosis. More than five years ago, Prolia (denosumab) was approved and, according to the company's 2015 annual financial report, now commands worldwide sales of more than $1.3 billion. The drug binds to RANKL, a transmembrane or soluble protein essential for the formation, function and survival of osteoclasts, the cells responsible for bone resorption.
Also at the conference, Waltham, Mass.-based Radius Health Inc. reported results from a pre-specified subgroup analyses from its phase III ACTIVE trial. The results demonstrate that its investigational drug, abaloparatide-SC injection, reduced the risk of vertebral and nonvertebral fractures and increased BMD consistently in postmenopausal women with osteoporosis regardless of baseline patient characteristics.
Abaloparatide is a synthetic peptide analogue of human parathyroid-hormone-related protein. The 18-month international study in 2,463 postmenopausal women with osteoporosis is designed to evaluate the efficacy and safety of abaloparatide-SC 80 mcg in the reduction of risk of vertebral and nonvertebral fractures.
According to J.P. Morgan analyst Jessica Fye, the latest update "further strengthens abaloparatide's clinical profile. [The] presentation was met with enthusiasm among the KOLs and physicians in the audience, especially with respect to abalo's impact on nonvertebral fractures. We see high probability of approval given the strength of abalo's clinical profile."
At the end of March, Radius submitted a new drug application to the FDA for abaloparatide-SC. If approved, the compound would be the first new bone anabolic in the U.S. since 2002, the company said.
With their late stage therapies, Amgen and Radius are looking to capture a sizeable chunk of the estimated $40 billion global market for osteoporosis drugs. (See BioWorld Today, Feb. 23, 2016.)
OTHER CONTENDERS
There are other contenders, too. Sermonix Pharmaceuticals LLC, for example, a specialty pharmaceutical company focused on the development and commercialization of emerging late-stage women's health products, said it will deliver an oral presentation related to its investigational drug, lasofoxifene, at the World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Disease this week. The investigational drug is in phase III development being compared to Evista (raloxifene, Eli Lilly and Co.) in preventing bone loss. The results, the company said, coupled with the known anti-fracture effectiveness of the drug, suggest that lasofoxifene is an attractive treatment option for women with or at risk for postmenopausal osteoporosis.
Lasofoxifene is a selective estrogen receptor modulator and has demonstrated reduction in both vertebral and nonvertebral fractures in postmenopausal osteoporosis.
According to David Portman, CEO of Sermonix, "This trial demonstrated greater bone mineral density changes as well as a nearly twofold higher rate of response, defined as no loss of BMD from baseline at 24 months, compared to raloxifene."
Bone Therapeutics SA, of Gosselies, Belgium, has an autologous bone cell product, Preob, in phase II for severe osteoporosis. The company reported the 12-month efficacy results from the first cohort of seven patients, which demonstrate positive effects on pain and osteoporosis blood markers of a single administration of Preob. Analysis of the profile of bone markers in the blood showed a dual trend: a decrease (of more than 25 percent) of bone resorption markers, while bone formation markers were either unaffected or even slightly increased, and, in a later-phase of the 12-month follow-up, a continuous increase in bone formation markers was observed with a moderate increase in bone resorption markers. Those preliminary results thus seem to indicate that a single administration of Preob progressively stimulates bone remodeling.
In July 2015, Menlo Park, Calif-based Corium International Inc. reported positive top-line interim results from its phase IIa study designed to determine the pharmacokinetics, pharmacodynamics and safety and tolerability of its microcor transdermal system for the rapid delivery of a treatment for osteoporosis. The product delivers human parathyroid hormone, or hPTH(1-34) (known as teriparatide), a peptide that has been clinically proven to stimulate formation of new bone and reduce the risk of fractures. The results, the company said, provide a strong foundation for moving the product further.
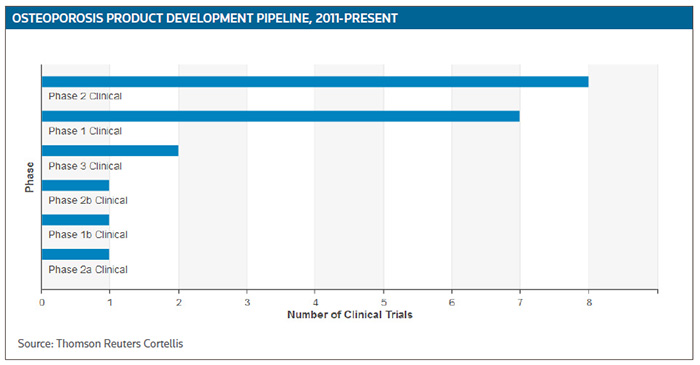
According to Cortellis Clinical Trials Intelligence, there are more than 60 trials currently recruiting patients for osteoporosis studies, including about 20 percent at the mid- to late trial stage. (See Osteoporosis Product Development Pipeline, below.)
All Portfolio
MEDIA CENTER
-
The RMI group has completed sertain projects
The RMI Group has exited from the capital of portfolio companies:
Marinus Pharmaceuticals, Inc.,
Syndax Pharmaceuticals, Inc.,
Atea Pharmaceuticals, Inc.

