
FDA has a backlog of 1,000 foreign plants that have never been inspected despite progress, GAO report finds
Print
23 January 2017
Eric Palmer / Fierce Pharma
The FDA in the last eight years has nearly doubled the number of annual foreign plant inspections it conducts, with India and China seeing the largest numbers of inspectors. But the agency still has a huge backlog of foreign plants that have never been seen and is struggling to fill foreign office jobs.
Those are among findings of a new Government Accountability Office report. With 40% of generic drugs and 80% of APIs coming from foreign countries, the GAO concluded that the FDA needs to do a better job of measuring the impact of its efforts on the U.S. drug supply.
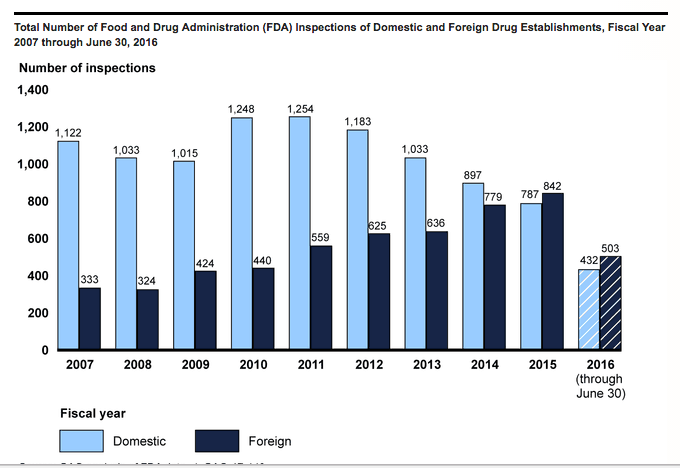
After problems with the safety of foreign-made meds came to light in the last decade, the FDA under former Director Margaret Hamburg steadily made improvements in foreign production oversight. According to the 63-page report, with more offices overseas, it was able to conduct 841 foreign inspections last year compared to 440 in 2010. Of those, 204 were in India and 129 in China, more than in other foreign countries.
The FDA has dramatically reduced its catalog of uninspected foreign plants that ship to the U.S.—from 64% in 2010 to 33% today. But about 1,000 still have never seen a U.S. inspector. The agency’s plan is to get to all of those in the next three years.
Many inspections are conducted by its U.S. staff, but the report said that as of July 2016, the FDA had 29 staff members assigned to work in the foreign offices, including a director and deputy director in each office. Some staff are also doing international work that is not part of the pharma inspection process, as well as local employees not counted in the numbers. But the report pointed out that 46% of the foreign offices' authorized positions were vacant as of July.
The report said that the FDA is making progress on its strategic planning for its offices in China, Europe, India and Latin America. It is measuring its success now in the numbers of inspections conducted, but the GAO believes it needs better measures of its efforts. In general recommendations, it said in addition to the numbers of inspections, the FDA needs to track things like import alerts and warning letters so it can get a better feel for how well its efforts are paying off.
The FDA is in the process of taking steps to use that kind of data to determine which plants need the most oversight. The Office of Pharmaceutical Quality was initiated by CDER Director Janet Woodcock in 2015 with plans to establish benchmarks to grade facilities so the FDA can determine which plants need to be more closely monitored. The quantitative information will replace the more general ideas the agency has had about the state of manufacturing quality.
The agency also combined the once-disparate oversight for generic versus branded drugs, and preapproved versus marketed. Now, teams of experts follow drugs through their life cycle, a process that should be more consistent for the agency and for drugmakers. The agency is also working more closely with agencies like the European Medicines Agency, Health Canada and others to share info and keep better tabs on companies in far-flung places.
All Portfolio
MEDIA CENTER
-
The RMI group has completed sertain projects
The RMI Group has exited from the capital of portfolio companies:
Marinus Pharmaceuticals, Inc.,
Syndax Pharmaceuticals, Inc.,
Atea Pharmaceuticals, Inc.

