
Raindrop Receives FDA Approval
Print
05 October 2016
Maria Scott, MD / Ophthalmology Management
A new corneal inlay changes the shape of the cornea to improve vision
 This year, I celebrated my20th anniversary of laser vision correction. I first needed glasses for myopia when I was 12, but only wore them in emergencies because I didn't want to look “nerdy.” At age 16, I got gas permeable lenses, which I loved, and later, laser vision correction, because I hated wearing glasses.
This year, I celebrated my20th anniversary of laser vision correction. I first needed glasses for myopia when I was 12, but only wore them in emergencies because I didn't want to look “nerdy.” At age 16, I got gas permeable lenses, which I loved, and later, laser vision correction, because I hated wearing glasses.
At the time of my surgery, I was young, and presbyopia was something that happened to "old" people. Still, I hedged my bets a little and targeted -0.5 in my nondominant eye.
In the lane, I would politely listen to middle-aged men and women complain that life was ending because they could no longer read without glasses. At the time, I thought to myself, What’s the big deal? Get a pair of glasses and be done with it. Then one day it happened to me.
I couldn't read black writing on a blue background at the theater. I thought, Oh no — life is ending! All my patients were right — this IS a big deal. For a while, I squinted or borrowed my husband's readers. But as time went on, I realized I couldn't go anywhere without them. Today, I usually wear a contact lens for monovision. But I have to admit that I sometimes forget I'm wearing it and I sleep in it. I wake up with incredible guilt and fear of getting an infection.
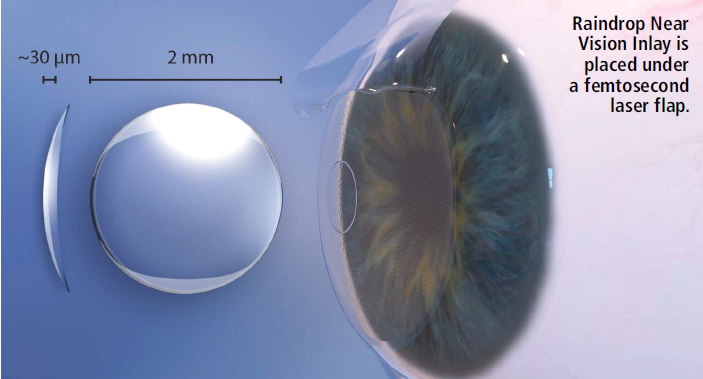
It is with this background that I share with you the exciting news that this past June, the FDA approved Revision Optics Raindrop corneal inlay. The swift, 9-month approval process surprised many, including the company. The approval of Raindrop marks the first time the FDA approved a device that changes the shape of the cornea to improve vision.
Promising Results
The Raindrop Near Vision Inlay approval was supported by a study that demonstrated clinically significant improvement in near vision acuity in subjects with age-related loss of near vision. The study involved 373 patients treated at 11 U.S. investigational sites. On average, patients experienced an improvement of five lines of near vision between their pre-operative examination and their 24-month post-op visit. Study participants also experienced an average improvement of 2.5 lines of intermediate vision; however, this was not a study endpoint. Although there was an average of 1.2 lines of decrease in distance vision in the treated eye, there was no change in distance vision binocularly. Refractive stability was achieved at 6 months.
Post approval, Revision Optics is continuing to follow the cohort for an additional 2 years. In addition, the company will enroll 528 eyes in the commercial setting and track them or 5 years.
Indications and Side Effects
According to the Approval Order Statement, "This device is indicated for intrastromal implantation to improve near vision in the nondominant eye of phakic, presbyopia patients, 41 to 65 years of age, who have manifest refractive sphericalequivalent +1.00 diopters to -0.5 diopters with less than or equal to 0.75 diopters of refractive cylinder, who do not require correction for clear distance vision, but do require near correction of +1.5 diopters to +2.50 diopters of reading add." Raindrop is a biocompatible hydrogel designed to be implanted under a femtosecond-laser-created corneal flap onto the stromal bed of the cornea and centered over the lightconstricted pupil of the nondominant eye during a 10-minute procedure. By reshaping and steepening the central curvature of the cornea, it provides a zone of increased power for focusing on near objects, resulting in improvement of near vision. It is 77% water, and has a refractive index similar to the cornea and a light transmission of 99.7% in the visual spectrum.
Because it is transparent, it does not restrict light. If necessary, it can be removed after lifting the flap.
The inlay is meniscus-shaped with an anterior curvature of 8.53 mm, a posterior curvature of 10 mm, a diameter of 2 mm, a central thickness of 32 microns, and an edge thickness of 12 microns. Raindrop provides no optical power, as its index of refraction is similar to that of the cornea. It is preloaded into an inserter and packaged in a glass vial.
Based on the improved results of a subgroup of 133 patients, recommendations for use include: targeting the flap depth to 30% of central corneal thickness with a minimum depth of 150 microns, and a minimal stromal bed thickness of 300 microns and a flap diameter of 8 mm or greater.
In this subgroup, no subjects experienced a loss of greater than one line of vision.
At 24 months, pain, foreign body sensation, light sensitivity, tired eyes, discomfort, glare, and halos were mild or absent in 99% of patients in the subgroup. Dryness at 24 months was mild or absent in 96% and moderate in 4% of subjects. Other potential side effects, many similar to LASIK, include: decreased contrast sensitivity in the implanted eye; infection; inflammation; decrease in best-corrected distance vision; ectasia; scarring; epithelial ingrowth; extrusion; shifted, or misaligned flap; epithelial defects or recurrent corneal erosions; inflammation, such as diffuse lamellar keratitis, corneal melt, or decompensation requiring corneal transplant; increased intraocular pressure related to use of steroids; and asecond surgical intervention.
Contraindications
The Raindrop inlay is contraindicated in patients who have the following:
• Corneal thickness that does not allow for a minimum of
300 microns of stromal thickness below the flap
• Abnormal corneal topography of the eye to be implanted
• Active eye infection or inflammation
• Active autoimmune or connective tissue disease
• Severe dry eye syndrome
• Keratoconus, or is a keratoconus suspect
• Recent herpes eye infection or problems resulting from a previous infection
• Uncontrolled diabetes
• Uncontrolled glaucoma.
A New Option
The surgical technique of placing the Raindrop corneal inlay is similar to LASIK. It provides great near results early in the post-op period with minimal compromise in distance vision. There are consistent visual outcomes independent of age and one inlay fits all. It provides a full range of continuous vision during the day and night without unwanted photic phenomena. The inlay is 100% removable with a low incidence of unwanted symptoms, such as glare and halos.
Jeffrey Whitman, MD, president and chief surgeon of Key-Whitman Eye Center in Dallas, who was involved in the clinical trial, says, “I performed almost 90 inlays in the FDA trial, and these patients are some of the happiest I have seen in my practice. If you can do LASIK, you can insert the Raindrop. No extra equipment is needed. If you follow the company’s recommended best practices, it is easy to achieve good outcomes. My first implantations post
approval are all J1 or J2 at 1 week.”
Revision Optics is also studying Raindrop in post-cataract surgery patients. The study began last year with a target enrollment of 400 patients. In my opinion, this is a huge market with few current solutions.
References & Resources
1. Whitman J, Hovanesian J, Steinert RF, Koch D, Potvin R. Through-focus performance with a corneal shapechanging inlay: one-year results. J Cataract Refract Surg 2016;42(7):965-971.
2. FDA. Premarket Approval: Raindrop Near Vision Inlay. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P150034
3. OIS News. Is surprising Raindrop approval a sign of a friendlier FDA? Available at: http://ois.net/is-surprisingraindrop-approval-a-sign-of-friendlier-fda/
INNOVATION
Maria Scott, MD, is founder and medical director of Chesapeake Eye Care and Laser Center and medical director of TLC Laser Eye Center Annapolis. She is a member of the OOSS Board of Directors.
All Portfolio
MEDIA CENTER
-
The RMI group has completed sertain projects
The RMI Group has exited from the capital of portfolio companies:
Marinus Pharmaceuticals, Inc.,
Syndax Pharmaceuticals, Inc.,
Atea Pharmaceuticals, Inc.

