
2015: A banner year for personalized medicine
Print
27 January 2016
Emma Van Hook / PhRMA
2015 was a record year for personalized medicine approvals, according to a new analysis from the Personalized Medicine Coalition (PMC). This news confirms the growing role of personalized medicine as an approach to treatment that can improve outcomes for patients and also create important efficiencies in the health care system. Personalized medicine is an emerging field of medicine that uses diagnostic tools to identify specific biological markers to help assess which medical treatments and procedures will be best for each patient. Personalized medicine also takes into account patients’ medical history, circumstances and values in developing targeted treatment and prevention plans.
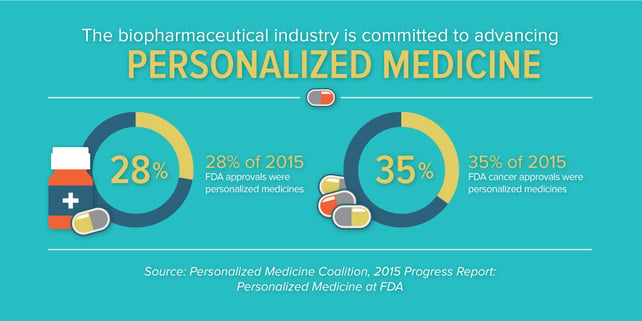
In what has already been noted as a banner year for new medicines, with 45 novel new drugs approved in 2015, the new analysis indicates that 28 percent of novel new drugs approved by the Food and Drug Administration in 2015 were personalized, or precision, medicines. This continued growth from the year before, where 21 percent of approvals were found to be personalized medicines, is reflective of the rapid progress we’re seeing in advancing targeted treatments for patients and affirms that personalized medicine has become an established approach to fighting a range of diseases. Previously, there were just a handful approved each year.
“The new analysis today affirms the biopharmaceutical industry’s commitment to developing personalized medicines. The continual increase of approved targeted therapies indicates where the science, research and the industry are all heading,” noted Edward Abrahams, president of PMC.
In recent years, we’ve seen tremendous advances in targeted therapies for diseases like cystic fibrosis, hepatitis C and many other conditions. But, without a doubt, we’ve seen the greatest transformation in oncology. A growing understanding of the molecular underpinnings of what was once referred to as a singular disease is enabling targeted therapeutic approaches for many forms of cancer. In 2015, 35 percent of new cancer medicines were found to be personalized medicines.
Some of the personalized medicine highlights from 2015 include:
- Two new medicines for patients with different forms of non-small cell lung cancer;
- A new combination therapy for patients with cystic fibrosis;
- Two new medicines to help patients with a difficult-to-treat form of high cholesterol; and
- A new targeted therapy for melanoma.
America’s biopharmaceutical companies have long been committed to accelerating the development of personalized medicines. The pipeline has never been more promising, with more than 40 percent of compounds in development having the potential to be personalized medicines. In cancer, that number is ever greater with 73 percent of cancer medicines in the pipeline potentially personalized.
The era of targeted therapy is rapidly transforming care for patients; we’re eager to see what 2016 will bring.
All Portfolio
MEDIA CENTER
-
The RMI group has completed sertain projects
The RMI Group has exited from the capital of portfolio companies:
Marinus Pharmaceuticals, Inc.,
Syndax Pharmaceuticals, Inc.,
Atea Pharmaceuticals, Inc.

